INSIGHT: A Scalable Isothermal NASBA-Based Platform for viral pathogens
Archive Page
This page is maintained as a historical record and is no longer being updated.
Technology
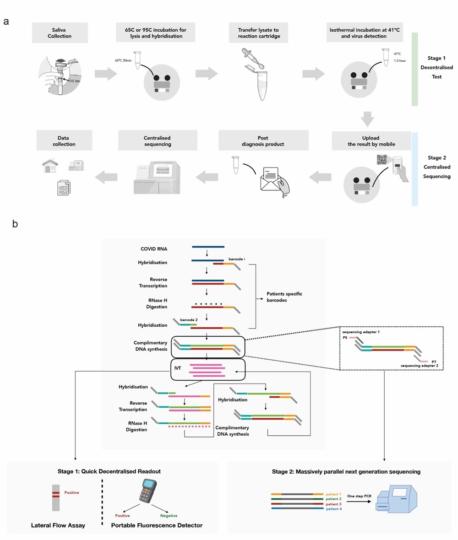
INSIGHT (Isothermal NASBA-Sequencing based HIGH-throughput Test) is a two-stage testing strategy for viral pathogens, including COVID-19. It uses a combination of an isothermal NASBA reaction and next generation sequencing. The first stage involves isothermal amplification of viral RNA whilst simultaneously incorporating sample-specific barcodes into the amplification product. Fluorescent and dipstick-based viral RNA detection can be consistently achieved at 10-100 copies per 20 μl reaction. The second stage pools post-amplification barcoded products from multiple samples for scalable sequencing that could be centralised, to further improve the accuracy of the test in a massively parallel way. This two-stage testing strategy is suitable for further development into a home-based or point-of-care assay and can be scaled to the population level.
Advantages
- Versatile: The test can be developed into a low-cost POC or home-based test.
- Scalable: Point of care (POC) test can be adjusted to deliver large scale testing at the population level using Next Generation Sequencing technologies.
- Simple to use: This two-stage test will work with samples taken from saliva, nasal and/or throat swabs.
- Rapid and sensitive: Detects down to 10 copies of the virus in 1-2 hours.
- Multi-Purpose: The test can be adjusted to detect other viral pathogens.
- Cost-Effective: Compared to alternatives on the market, this method does not require the use of more expensive and complicated thermocyclers. This makes it attractive for use in areas with minimal infrastructure.
- Dual detection method: Fluorescent and dipstick-based detection can be consistently achieved with accuracy.
- Validated on patient samples
Detail
Applications/Context
- Rapid detection of viral RNA (including COVID-19) from saliva or throat/nasal swabs using a point of care test (POC).
- Identification of viral RNA from pooled barcoded patient samples that can be scaled up to the population level.
Comparable Technologies
Existing point of care (POC) testing devices that work with nasal and throat swabs, already provide rapid test results in critical settings such as hospitals. We believe our technology may help lower the cost of POC testing even further and simplify sample collection by adding saliva as an option. In addition, the sequencing based component of our testing strategy that pools multiple patient samples could help increase the scalability of NGS-based diagnostic approaches being considered.
Background
The COVID-19 pandemic has caused significant loss of life as well as widespread economic hardship across the world. Alongside effective vaccination programmes, rapid identification, quarantine, and treatment of infected individuals will allow governments to reopen societies without increasing rates of infection and mortality.
Intellectual property
Priority patent applications filed in UK. The Wellcome Sanger Institute is offering non-exclusive licenses to its IPR. Licenses will be free for research on, and diagnosis of COVID-19.
Download
 INSIGHT_flyer_APR21
INSIGHT_flyer_APR21
