Study reveals new genetic mechanism driving breast cancer
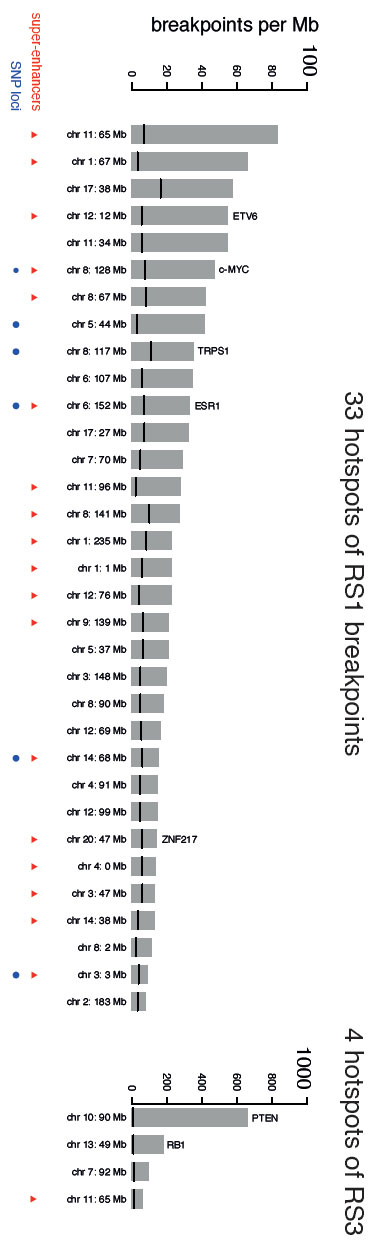
Researchers at the Wellcome Trust Sanger Institute have discovered ‘hotspots’ of mutations in breast cancer genomes, where mutations thought to be inactive ‘passengers’ in the genome have now been shown to possibly drive further cancerous changes. Reported in Nature Genetics today (23 January 2017), the study found 33 mutation hotspots, which act like backseat drivers for cancer development.
The study analysed 560 breast cancer genomes from patients from around the world, including the USA, Europe and Asia. It looked at very specific types of mutations – called tandem duplications. These occur when a piece of DNA is copied and then reinserted in the DNA next to the original piece, creating extra copies of that part of the DNA in the genome.
The researchers found 33 places in the breast cancer genomes where these tandem duplications were found most often, recurrently in many different women.
Cancer is triggered by a small handful of ‘driver’ DNA mutations that actively change cells from being normal cells into cancer cells. Cancer cells also contain many thousands of ‘passenger’ mutations such as tandem duplications, which were previously thought to be inactive, or simply collateral damage. This study found that tandem duplications in these hotspots were possibly not benign, but led to further cancerous changes, creating new drivers in a cascade of genetic damage.
“DNA in breast cancers is heavily restructured by many tandem duplications. We were curious whether this mutational signature influences how cancer develops. From our research, it now looks like some of these tandem duplications are not just unimportant passenger mutations, but actually create new driver mutations for cancer.”
Dominik Glodzik First author on the paper from the Wellcome Trust Sanger Institute
Studying tumours with hotspot mutations will provide insights into how new backseat driver mutations shape cancer development, and whether they will respond to different treatments.
The researchers found that many of the tandem duplication hotspots were in important parts of the genome. These included genes that are known to be associated with driving breast cancer and also in special, regulatory areas of the genome known as super-enhancers that switch on multiple genes – damage in this area leads to uncontrolled activation of many genes. The study shows that there is probably a spectrum of drivers too – some can be very strong and some can be weaker – but all likely add together to ultimately make cancers more aggressive.
“Remarkably, we also found that these acquired hotspots for tandem duplications coincide with genome regions where we find not only inherited genetic changes that increase the risk of developing breast cancer, but also controllers of genes that specify the identity of breast epithelial tissue. This is a first report that demonstrates a link between the acquired and the inherited genetic changes that drive breast cancers.”
Professor Gerard Evan from the University of Cambridge
The study also looked at a small number of ovarian and pancreatic cancer genomes and found these also had hotspots of tandem duplications But in different places than the breast cancers, due to different genes being activated in diverse tissues. Further samples will be needed to get more information on ovarian and pancreatic cancers.
“This research has shown a new genetic mechanism for generating drivers in cancer, which we have found not only in breast cancers, but also other cancers. It is really important to identify and study tumours with this mutational signature of tandem duplications, because if it can create many new drivers, then it has potentially devastating consequences making tumours more aggressive.
“This is a mutational signature that should be a target for therapeutic intervention. Further study is needed to understand this mechanism, and for finding out whether tumours with tandem duplications at these hotspots could respond to certain treatments better than others.”
Dr Serena Nik Zainal, lead author from the Sanger Institute
More information
Funding
This work was supported by Wellcome and by the European Community’s Seventh Framework Programme.
Publications:
Selected websites
University of Cambridge
The mission of the University of Cambridge is to contribute to society through the pursuit of education, learning and research at the highest international levels of excellence. To date, 96 affiliates of the University have won the Nobel Prize. Founded in 1209, the University comprises 31 autonomous Colleges, which admit undergraduates and provide small-group tuition, and 150 departments, faculties and institutions. Cambridge is a global university. Its 19,000 student body includes 3,700 international students from 120 countries. Cambridge researchers collaborate with colleagues worldwide, and the University has established larger-scale partnerships in Asia, Africa and America. The University sits at the heart of one of the world’s largest technology clusters. The ‘Cambridge Phenomenon’ has created 1,500 hi-tech companies, 14 of them valued at over US$1 billion and two at over US$10 billion. Cambridge promotes the interface between academia and business, and has a global reputation for innovation.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
Wellcome
Wellcome exists to improve health for everyone by helping great ideas to thrive. We’re a global charitable foundation, both politically and financially independent. We support scientists and researchers, take on big problems, fuel imaginations and spark debate.


