Origin and evolution of Bordetella pertussis
The most comprehensive study to date of the family of bacteria that causes whooping cough points to more effective vaccine strategies and reveals surprising findings about the bacteria’s origin and evolution. The new results could alter public health strategies to control this respiratory disease, which kills 195,000 children worldwide each year.
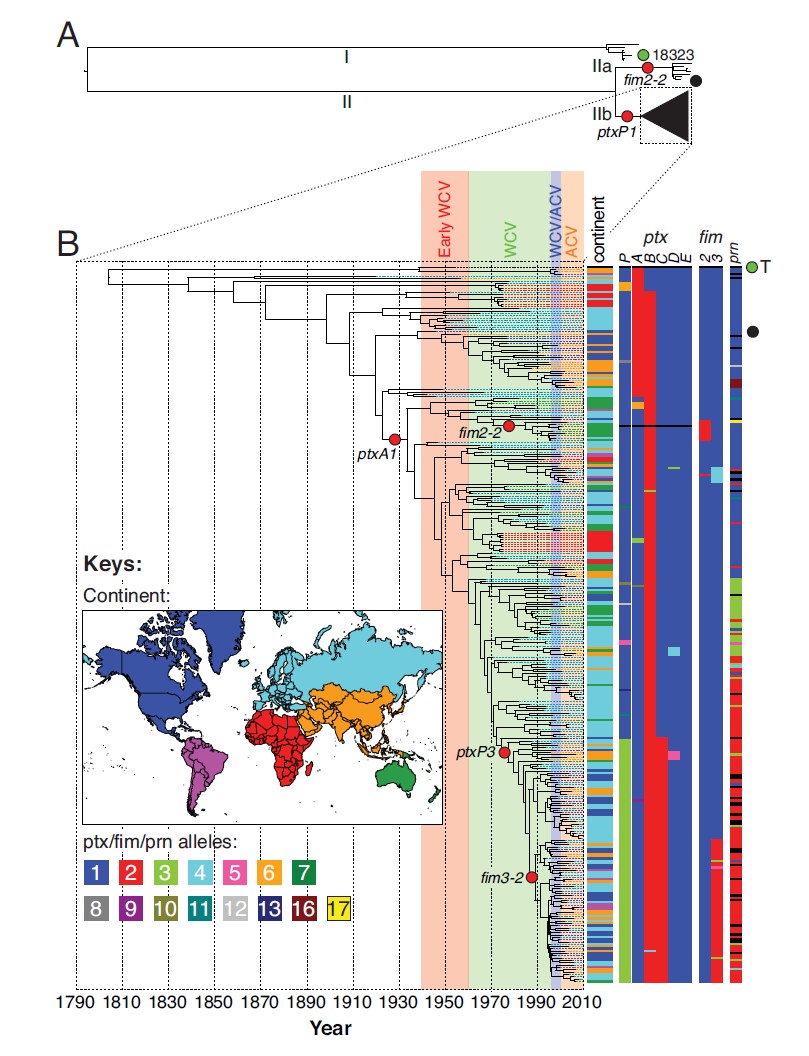
Genomic analysis of 343 strains of the Bordetella pertussis bacteria from around the world collected over the last 100 years illustrates how vaccination has shaped its evolution. Since its introduction across the globe between 1940 and 1960, vaccination has dramatically reduced rates of infection and loss of life from whooping cough. However, strategies used to date have not completely eradicated strains of the bacteria, instead leading to an increase in diversity.
“The scale of this study and the detailed family history of B. pertussis we are able to draw from it illustrate the journey this bacterium has taken since its emergence. By seeing how an organism escapes vaccination we can build better strategies to control and eradicate it.”
Dr Simon Harris A first author at the Wellcome Trust Sanger Institute
While researchers suspect that the diversity may be the result of lineages of the bacteria persisting in unvaccinated populations, resurgence of B. pertussis has also been observed between 2010 and 2012 in highly vaccinated populations such as Australia, the Netherlands, the UK and the USA. One reason could be the widespread switch to the use of acellular vaccines, which, though better tolerated than the original whole-cell vaccines, tend to produce more rapidly-waning immunity.
“To stem the rise of whooping cough without returning to whole-cell vaccines, which can be reactogenic, we need to employ strategic vaccination. This could include vaccinating mothers in pregnancy so babies are born with some level of protection and cocooning a newborn by vaccinating adults around it. In the long run, however, we need better pertussis vaccines.”
Dr Marieke Bart A first author from the National Institute of Public Health and the Environment, the Netherlands
As well as suggesting strategies for control in the future, the study revealed new findings about B. pertussis‘ past. Genetic analysis of the strains shows that whooping cough emerged and spread rapidly in humans in the late Middle Ages, around 500 years ago, not in the Neolithic period more than 10,000 years ago, as previously thought.
This finding resolves a puzzle that had troubled scientists for some time: despite characteristic symptoms and a high rate of mortality in unvaccinated children, records of whooping cough are entirely absent from ancient European literature. This new analysis, which places the emergence and rapid global spread of the infection in the human population within the last 500 years, is consistent with historical records that first identify the disease in Paris in 1578. The only earlier reference to a disease with similar symptoms has been found in a Korean medical textbook from the 15th Century.
“Over the past five years or so it has become clear that we have got our dating in bacteriology wrong. The mutation rate we were working with is probably two orders of magnitude out, so we thought the last common ancestor of these modern B. pertussis strains was tens of thousands of years old when, in fact, it is much younger than that.”
Professor Julian Parkhill A senior group leader at the Sanger Institute and a senior author of the study
More information
Funding
This work was supported by the Wellcome Trust (grant number 098051), the RIVM (SOR project S/230446/01/BV), and the National Health and Medical Research Council of Australia.
Participating Centres
A full list of participating centres can be found on the paper.
Publications:
Selected websites
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


