Human endometrial map uncovers hidden health clues
Listen to “Human endometrial map uncovers hidden health clues” on Spreaker.
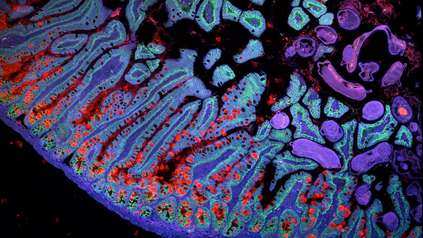
The most comprehensive map of the human endometrium, the inner lining of the uterus, has been created, uncovering diverse cell types and detailing the dynamic changes these go through during the menstrual cycle.
Encompassing the widest range of menstrual cycle phases ever mapped, the atlas reveals new insights into endometrium functioning, informing health research. Built by researchers from the Wellcome Sanger Institute, the Nuffield Department of Women’s and Reproductive Health at the University of Oxford, and collaborators, it could help study, understand, and possibly treat conditions such as endometriosis in the future.
The study, published today (28 August) in Nature Genetics, is part of the wider Human Cell Atlas project1, which is mapping all cell types in the body to transform understanding of health and disease. By linking to genetic variants known to increase the risk of endometriosis, the researchers found two types of immune cells and two types of stromal cells that are potentially involved in the condition, highlighting new avenues for future research.
The Human Endometrial Cell Atlas is now publicly available in an easy-to-access interactive format and is expected to be a valuable central resource for researchers studying the endometrium and be used to inform and develop more effective laboratory models2.
Having an in-depth map of the endometrium allows researchers to gain insight into unique cells and interactions not found elsewhere in the body, and better understand tissue changes during the menstrual cycle, including the endometrium’s ability to regenerate without scarring.
The endometrium is crucial for human reproduction, supporting pregnancy if a fertilised egg is implanted. If no implantation occurs, it sheds and rebuilds itself each month without scars. This ever-changing tissue goes through complex and dynamic changes across the menstrual cycle, making it extremely hard-to-study.
Endometrial conditions impact millions of people around the world. Endometriosis, a chronic condition where endometrial-like cells grow outside of the uterus, is the second most common gynaecological condition in the UK3. Among other symptoms, it can cause debilitating pain and fertility issues. There is currently no cure, and the cause of the condition is unknown3.
Previous studies investigating the human endometrium and uterus have been able to build a limited picture, but never one that captured all the stages that the endometrium goes through across the menstrual cycle4.
This new Endometrial Cell Atlas, led by researchers at the Wellcome Sanger Institute and the Nuffield Department of Women’s and Reproductive Health at the University of Oxford, generated new data on 74 endometrial samples5. They harmonised the data with existing single-cell data from 47 people4. In total, the atlas contains data for around 626,000 cells from 121 people, including from individuals with and without endometriosis, both during natural menstrual cycles and when taking hormonal contraception.
They found multiple new cell types that are only present in certain phases of the menstrual cycle, depending on the hormone levels. A cellular response to hormone levels is essential for menstrual cycle progression and fertility, and these cells could present promising therapy targets for conditions linked to hormone disruption, such as fertility conditions.
The team uncovered interactions involved in the scarless regeneration of the endometrium between immune cells called macrophages, a type of connective tissue cell known as stromal cells, and blood vessel cells. Understanding how these pathways may be disrupted in common menstrual conditions, such as abnormal bleeding where the lining continues to shed, could help find new interventions.
While there were no notable differences in the number of cell types between those with endometriosis and those without, there were small differences in the proportion and gene expression of some cells in those with endometriosis. These findings are in line with previous work, but the atlas offers a more detailed view of specific cell types that may be dysregulated in endometriosis.
To investigate the impact of genetic variants that have previously been linked to endometriosis, the researchers combined their in-depth cellular map of the endometrium with a large genome-wide association study6.
The team highlighted four cell types that are most likely dysregulated by these genetic changes. They also found particular signalling pathways between some stromal cells and structural cells were dysregulated in those with endometriosis. These signalling pathways are known to be needed for menstrual cycle progression.
The study highlights that these cell types and signalling pathways could be involved in endometriosis and may support future research investigating how genetic changes are linked to this condition.
“Having this in-depth and large-scale genomic resource on the endometrium is invaluable if we are ever going to fully understand how the endometrium functions in health and what goes wrong in conditions such as endometriosis. Developing a non-invasive diagnostic test and effective treatment for this debilitating condition has been a priority for clinicians, researchers and those with endometriosis worldwide. While further research and validation is needed, our study suggests that certain cells and pathways are dysregulated in endometriosis and if replicated in additional studies, they could be potential diagnostic and therapy targets in the future.”
Dr Magda Marečková, co-first author from the Nuffield Department of Women’s and Reproductive Health at the University of Oxford, and the Wellcome Sanger Institute
“Creating an integrated Human Endometrial Cell Atlas transforms all the data into ‘one language’ that researchers worldwide can speak. We hope that this atlas is a key stepping stone towards building a future endometrial atlas that includes information from across the entire lifespan and all health conditions. Further studies that gather additional health information, such as if the individual has a regular period or a previous pregnancy, as well as genomic data could help build the atlas and further investigate factors that might play a role in uterine health and the development of endometrial conditions.”
Dr Luz Garcia-Alonso, co-first author from the Wellcome Sanger Institute
“The human endometrium has been largely neglected in large-scale cellular studies of different parts of the body. Having a large single-cell human endometrial atlas, freely available, which will continue to be expanded, will enable important new research in the understanding and treatment of diseases specific to women and those born with a uterus, such as endometriosis.”
Professor Krina Zondervan, co-senior author from the Nuffield Department of Women’s and Reproductive Health at the University of Oxford
“The human uterus is a dynamic and poorly understood part of the body, which holds key information that could be used to treat a range of conditions. Understanding the impact of hormones on the endometrium and being able to map all the changes throughout the menstrual cycle is something that has not been possible previously, and would not have been achievable without those who generously donated endometrial tissue to research. We have been able to reveal unique interactions and cells that can inform research models, investigate the possible causes of diseases, and create a resource that can be used freely worldwide to help develop new therapies for those living with endometrial conditions.”
Dr Roser Vento-Tormo, co-senior author from the Wellcome Sanger Institute
More information
The researchers would like to thank all the participants involved, and this research would not have been possible without them.
The freely available integrated Human Endometrium Cell Atlas can be found here: https://www.reproductivecellatlas.org/endometrium_reference.html
The team conducted single-cell and spatial genomic analysis to map every cell in a sample, where it is found, and how it communicates with its environment. The researchers have also developed a new tool to integrate data into the atlas, as well as producing training materials to ensure that future studies can continue to be added to this resource.
- This study is part of the Human Cell Atlas (HCA), an international collaborative consortium which is creating comprehensive reference maps of all human cells — the fundamental units of life — as a basis for understanding human health and for diagnosing, monitoring, and treating disease. The HCA is likely to impact every aspect of biology and medicine, propelling translational discoveries and applications and ultimately leading to a new era of precision medicine. The HCA was co-founded in 2016 by Dr Sarah Teichmann, then at the Wellcome Sanger Institute (UK) and Dr Aviv Regev, then at the Broad Institute of MIT and Harvard (USA). A truly global initiative, there are now more than 3,500 HCA members, from 101 countries around the world. https://www.humancellatlas.org
- The breadth of information in the atlas can be used to develop effective laboratory models to study the endometrium. Current models have noted cells in laboratory models that are not seen in human tissue, and having the information on cell communication could inform how samples and organoids are stored to minimise unwanted interactions.
- Endometriosis Facts and Figures, Endometriosis UK. Available at: https://www.endometriosis-uk.org/endometriosis-facts-and-figures
- Existing studies often have different processes and labelling systems which can lead to difficulties when combining data. This latest research standardised all the single-cell data from six previous studies that included samples from 41 people. The team also developed a new tool to guide dataset integration, cell annotation and ensure reproducibility across studies, to allow new datasets to be added in the future.
- Samples were collected from donors during necessary exploratory surgeries to investigate whether patients had endometriosis. The team conducted single-cell and spatial genomic analysis to map every cell in a sample, where it is found, and how it communicates with its environment.
- This genome-wide association study meta-analysis included 60,674 cases and 701,926 controls and identified 42 significant genetic changes associated with endometriosis. Full publication: N. Rahmioglu, S. Mortlock, M. Ghiasi, et al. (2023) The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nature Genetics. DOI: 10.1038/s41588-023-01323-z
Publication:
M. Marečková, L. Garcia-Alonso, M. Moullet, et al. (2024) An integrated single-cell reference atlas of the human endometrium. Nature Genetics. DOI: 10.1038/s41588-024-01873-w
Funding:
The research was funded in part by Wellcome, the Chan Zuckerberg Initiative, the John Fell Fund, the Medical Research Council, and the European Union’s Horizon 2020 research and innovation programme HUTER. A full acknowledgement list can be found in the publication.