Cellular changes as lung damage worsens in COVID-19 revealed
Listen to this news article:
Listen to “Cellular changes as lung damage worsens in COVID-19 revealed” on Spreaker.
Changes in the cellular response during lung damage caused by SARS-CoV-2 – the virus behind COVID-19 – have been uncovered. Researchers reveal distinct phases where waves of immune responses give way to lung fibrosis – scarring of the lungs – in severe COVID-19.
As part of the UK Coronavirus Immunology Consortium (UK-CIC), researchers from the Wellcome Sanger Institute, Imperial College London, Newcastle University and Harvard University used a combination of cell mapping technologies to build a comprehensive understanding of the cellular response and lung tissue changes instigated during COVID-19. This study is part of the international Human Cell Atlas initiative to map every cell type in the human body.1
The research, published today (10 March) in Nature Communications, shows a new set of molecular markers that distinguish gradual stages of damage to alveoli in the lungs. The work opens the door to further analysis of cellular mechanisms underlying inflammatory responses in severe disease.
With over seven million deaths caused by COVID-19 worldwide, the SARS-CoV-2 virus continues to spread, with the most common cause of death being respiratory failure.2,3 Alveoli are balloon-shaped air sacs in the lungs where the blood and lungs exchange oxygen and carbon dioxide during breathing. Lung damage from COVID-19 is known as diffuse alveolar damage (DAD).
DAD shows distinct pathological features as it worsens from early-stage to late-stage. Whilst previous studies document an expanded immune response in late DAD, knowledge of the cellular and molecular differences between early and late stages of alveolar damage has been limited. It is important for researchers to understand these differences so therapies can be developed in order to prevent patients from developing severe COVID-19.
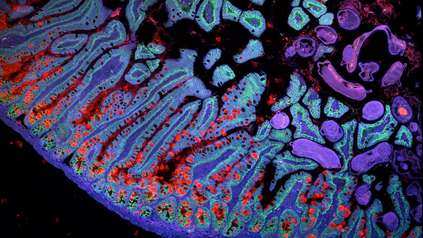
In a new study, they combined single cell RNA sequencing data and spatial transcriptomics data from post-mortem lung tissue samples of COVID-19 patients.4,5 The combination of these two methods allowed the researchers to identify the gene activity, molecular biomarkers and interactions between cells associated with early versus late-stage DAD.
In early-stage DAD, the researchers identified activity in genes associated with protective inflammatory responses, such as an increased expression of interleukin genes – proteins that regulate the immune response. They also found upregulation of metallothionein-related genes, which are thought to protect cells from toxic high-metal content. Surprisingly, the researchers also saw different waves of macrophages, a key immune cell type in COVID-19, as DAD progressed from early to late stage.
In late-stage DAD, the researchers saw an increase of markers associated with lung fibrosis – a lung pathology that causes scarring and stiffness in lung tissue, making it difficult to breathe.
The team also noted changes in the regulation of several genes that are involved in fibrinolysis. Fibrinolysis is a normal process that involves the breaking down of blood clots. They demonstrated the gene, SERPINE1 to be a key player in the dysfunction of fibrinolysis as seen in lung cells infected with SARS-CoV-2. They also note this gene is upregulated more in early DAD compared to late DAD and is promoted via signals from disease-associated macrophage populations.
The researchers hope that a better understanding of the cellular and molecular mechanisms underlying progressive lung damage during COVID-19 will inform future studies and ultimately lead to therapies that benefit patients before they develop severe disease.
“Our study allowed us to build a more thorough picture of how our lungs respond to the SARS-CoV-2 virus. We identified a group of new cell types that change between early and late stages of lung damage. We also identified sub-groups of immune cells, called macrophages, that start to accumulate in very early stages of infection, and how they shift to different groups as the disease worsens. Our results provide a more detailed understanding about a disease that affects people worldwide.”
Dr Jimmy Tsz Hang Lee, co-first author and a postdoctoral researcher at the Wellcome Sanger Institute
“One of the hallmarks of severe disease is clotting in blood vessels of the lung. Here, we identified the factors and the specific cells driving this process, suggesting that there is an accumulation of blood clots due to a defect in mechanisms that break them down. This provides a potential target for resolving these blockages and restoring blood flow in the lung tissue.”
Dr Sam Barnett, co-first author and postdoctoral researcher at Imperial College London
“The combination of single cell data and spatial transcriptomic data allows you to immediately identify the genes and the molecular biomarkers that are enriched in early versus late stages of alveolar damage. The integrated method provides a detailed understanding of molecular processes underlying COVID-19, which is a huge leap for understanding a virus that is still impacting millions of people.”
Dr Martin Hemberg, Associate Professor at Brigham and Women’s Hospital and Harvard Medical School, and former Group Leader at the Wellcome Sanger Institute
“This is an excellent example of how collaboration across centres and across countries allows us to generate an in-depth understanding of how COVID-19 caused such catastrophic lung disease in some people. In Newcastle, we assembled one of the world’s largest collections of lung samples collected from patients who died from COVID-19 and with our collaborators have applied cutting edge technology that reveals exactly how the immune system contributed to severe lung damage over time. Armed with this information we will be better able to identify potential treatments to combat any future respiratory viral pandemics.”
Professor Andrew Fisher, Professor of Respiratory Transplant Medicine at Newcastle University
“This successful study has been the result of a strong culture of team-science. Interdisciplinary collaborations bring in a diversity of approach that is key to exploring disease effectively.”
Dr Michela Noseda, Reader in Cardiovascular Science at the National Heart and Lung Institute, Imperial College London
“When the most common death from COVID-19 is respiratory failure, we have a duty to investigate what causes the underlying lung damage and how we can intervene. Our study provides new in-depth detail on the dynamic nature of tissue damage in the lungs, which we hope influences change one day, whether that is for further research or the development of therapies, to help those who contract COVID-19.”
Dr Omer Ali Bayraktar, Group Leader at the Wellcome Sanger Institute
More information
Footnotes
- The HCA is an international collaborative consortium whose mission is to create comprehensive reference maps of all human cells—the fundamental units of life—as a basis for understanding human health and for diagnosing, monitoring, and treating disease. The HCA community is producing high quality Atlases of tissues, organs and systems, to create a milestone Atlas of the human body. More than 3,500 HCA members from over 100 countries are working together to achieve a diverse and accessible Atlas to benefit humanity across the world. Discoveries are already informing medical applications from diagnoses to drug discovery, and the Human Cell Atlas will impact every aspect of biology and healthcare, ultimately leading to a new era of precision medicine. https://www.humancellatlas.org
- World Health Organisation Data. WHO COVID-19 dashboard. Available at: https://data.who.int/dashboards/covid19/deaths?n=o [Last accessed: February 2025]
- Ketchum, S. W. et al. (2021) Causes and Circumstances of Death among Patients Hospitalized with COVID-19: A Retrospective Cohort Study. Annals of the American Thoracic Society. Doi: 1016/S1473-3099(20)30120-1
- The single cell data allow researchers to describe cell types, cell states and gene expression programs associated with disease in an unbiased manner. Spatial transcriptomics data allows the computational mapping of cell types and their locations.
- Human lung samples from patients who died with severe COVID-19 were obtained from UK based biobanks. Samples were obtained from Westminster Public Mortuary (Michael Osborn & Brian Hanley) and Imperial College Healthcare Tissue Bank, the Newcastle Hospitals CEPA Biobank and the ICECAP tissue bank at the University of Edinburgh.
Publication
Jimmy Tsz Hang Lee et al. (2025) ‘Integrated histopathology, spatial and single cell transcriptomics resolve cellular drivers of early and late alveolar damage in COVID-19.’ Nature Communications. DOI: 10.1038/s41467-025-56473-x
Funding
This research was part of the UK Coronavirus Immunology Consortium (UK-CIC). For full funding acknowledgements, please refer to the publication.