From gene to function
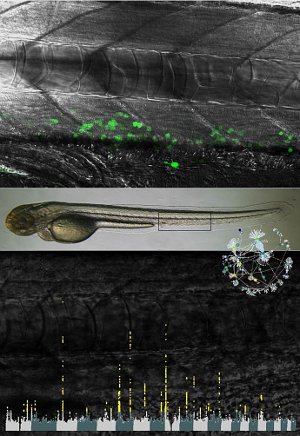
In a study into the genetics of blood cell formation, researchers have identified 68 regions of the genome that affect the size and number of platelets. Platelets are small cells that circulate in the blood and are key to the processes of blood clotting and wound healing.
In this genome-wide study, the team used a multidisciplinary approach to successfully identify new genetic variants involved in the formation of platelets and more importantly, defined the function of genes near these variants using a series of biological analyses.
Abnormally high or low platelet counts can lead to disease. An increase in the number of platelets, or an increase in their size can lead to an increased risk for thrombotic events, like heart attacks and strokes. A very low number of platelets or platelets that do not function well, increases the risk of bleeding.
“This is a detective story starting with the initial genetic discovery and allowing us to identify new genes that could contribute to platelet associated diseases. Our aim of this genome-wide meta-analysis study was to discover which genes control the size and number of platelets, to understand how these genes instruct blood stem cells to orchestrate every day the formation of billions of platelets and finally to investigate whether genes associated with heart attacks and strokes overlap with the genes that affect platelet formation.”
Professor Willem H Ouwehand Senior co-author at the University of Cambridge and NHS Blood and Transplant
In this collaborative study, the team first developed a prioritisation strategy that allowed them to identify and pinpoint the genes underlying the formation of platelets through biological annotations of these genes. This effort laid the foundation for the construction of a protein-protein interaction network that shows how the different genetic players interact. Finally, they analysed the role of the genes in model organisms and found their function to be conserved in evolution.
The researchers found the newly identified genes associated with platelet characteristics overlap with other genes implicated in inherited bleeding disorders. This genetic overlap suggests that this study may help discover new genes implicated in severe forms of bleeding disorders, providing evidence that the new findings will be significant in clinical research for improvements in the care of patients.
This study involved about 68,000 individuals from different ancestries (European, South & East Asian) making it the largest genome-wide meta-analysis study to be conducted globally on platelet number and volume.
“This is the largest dataset of this type ever produced, and yields a wealth of new exciting biological discoveries and insights into the genetic control of blood cell formation. Our findings will be relevant not only to better understand the mechanisms leading to the formation of blood cells, but also to pinpoint new genes involved in diseases with altered blood clotting.”
Dr Nicole Soranzo Senior co-author from the Wellcome Trust Sanger Institute
The team examined the role of the genes they identified in the fruit fly and zebrafish. They found that reducing the activity of one of these genes, ARHGEF3, in fish abrogates not only the production of platelets but also of red blood cells because the blood forming cells cannot capture iron. The study has shown that the human equivalent, ARHGEF3 gene is an important new regulator of the uptake of iron from the diet.
Tropomyosin 1 is a member of a family of genes already known to be involved in the regulation of muscle contraction and plays a role in heart disease. This study found a novel role for this well-known protein in platelet formation.
“This study provides a paradigm for how to successfully translate genome-wide association studies into function. We have shown that biologic and functional annotation can greatly enhance our ability to interpret genetic data.
“These genes could be used in the future as new targets to develop better and safer platelet inhibitors for treatments of patients with heart attacks or strokes.”
Dr Christian Gieger Senior co-author from the Institute of Genetic Epidemiology at the Helmholtz Center Munich
More information
Platelets are the second most abundant cell in the blood. Their main task is to survey the blood vessel wall for damage and to initiate its repair where required. A low platelet number increases the risk of bleeding. On the flip side, platelets also play a “darker” role after damage of the vessel wall they cause blood clots that may lead to heart attacks or strokes. An increase in the number of platelets or having big platelets increases the risk for these Number 1 killer diseases.
Anaemia caused ill-health in tens of thousands of individuals and the shortage of red blood cells is generally caused by a lack of iron.
This research involved 124 researchers from 13 different countries all over the world.
Funding
The study by the HaemGen consortium has been supported by a large number of fund providers, including AC and DLS are supported by the Wellcome Trust, the National Institute for Health Research for England, the Medical Research Council, the British Heart Foundation and NHS Blood and Transplant, the European Union, the EBI Industry programme and the US National Institutes of Health and many others. The study would not have been feasible without the generous free gift of blood samples and related information for research use by nearly 70,000 volunteers.
Participating Centres
Detailed list of participating centres found in the research paper.
Publications:
Selected websites
NHS Blood and Transplant
NHS Blood and Transplant (NHSBT) is a national NHS organisation with about 6,000 employees and responsibility for the collection of blood and platelets from non-remunerated volunteer donors and for the supply of organs, other tissues and blood stem cells for transplantation (visit www.nhsbt.nhs.uk) and for research and development in blood and tissues.
Every day about 7,000 donors make their ‘gift of life’ to provide NHS patients with units of safe blood. Similarly every day about 500 special platelet donors attend a clinic for two hours during which platelets are harvested from the blood of the donor by a process called apheresis. These concentrates of platelets are used for the treatment of cancer patients who lack platelets and for patients undergoing surgery who are bleeding. It is hoped that the research reported here will eventually result in the development of a method to produce platelets in the laboratory for the treatment of patients. To become a donor of blood or platelets, please: visit www.blood.co.uk or phone 0300 123 23 23
The British Heart Foundation
The British Heart Foundation (BHF) is the nation’s heart charity, dedicated to saving lives through pioneering research, patient care, campaigning for change and by providing vital information. But we urgently need help. We rely on donations of time and money to continue our life-saving work. Because together we can beat heart disease.
The Wellcome Trust Sanger Institute
The Wellcome Trust Sanger Institute is one of the world’s leading genome centres. Through its ability to conduct research at scale, it is able to engage in bold and long-term exploratory projects that are designed to influence and empower medical science globally. Institute research findings, generated through its own research programmes and through its leading role in international consortia, are being used to develop new diagnostics and treatments for human disease.
The Wellcome Trust
The Wellcome Trust is a global charitable foundation dedicated to achieving extraordinary improvements in human and animal health. We support the brightest minds in biomedical research and the medical humanities. Our breadth of support includes public engagement, education and the application of research to improve health. We are independent of both political and commercial interests.


